Over the past decades, IR has made significant advancements not only in thermal ablation technology but also in enhancing the safety of these procedures. Extensive research and clinical experience have demonstrated that thermal ablation can be performed with precision and safety, even in the most challenging anatomical locations. But what are the most challenging locations for thermal ablation and how have recent advancements addressed these difficulties?
During my talk on ECIO 2025, I will address the challenges encountered when performing thermal ablation in vicinity to the neural structures (spinal cord, motor and sensitive nerves).
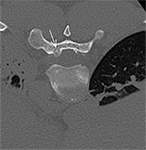
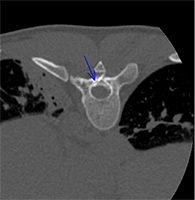
Neural tissues are highly susceptible to thermal injury due to their lipid-rich myelin sheath and limited regenerative capacity. The extent of injury depends on the extreme temperature reached, the total exposure duration, and lastly the tissue composition. Thermal damage can occur through protein denaturation, membrane disruption, ischemia due to vascular occlusion, and inflammatory responses that contribute to secondary neural injury. Effective neuroprotection strategies are essential to mitigate these risks and preserve nerve function. The primary goal when protecting a nerve from injury is to keep the juxtaneural temperatures between 10 and 45°C.
There are two main approaches to thermoprotection; passive neuroprotection and active neuroprotection. Passive thermoprotection techniques focus on monitoring the temperature or the electrical conductivity of the nerves, allowing intervention before permanent neural damage occurs.
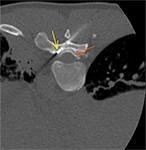
One of the most popular techniques of passive neuroprotection is temperature monitoring. Real-time thermometry with the use of thermal sensors (thermocouples) is readily applicable in everyday practice. The thermocouples are deployed in contact with the nerve at the location of the closest distance to the ablation zone. Whenever the temperature on the nerve is approaching the critical threshold (<10°C and >45°C), caution should be applied and active thermoprotection measures should be considered. Thermocouples (22-27G) are embedded into ablation devices, but independent systems can also be used to prevent excessive heating/freezing of the neural structures.
Biofeedback is another simple yet reliable passive thermoprotection method, but requires patient cooperation. Sensory biofeedback involves reporting pain in areas corresponding to the targeted nerve, while motor biofeedback assesses function through movement. Any decline in motor ability should trigger active neuroprotection.
For patients under general anaesthesia, electrostimulation of motor nerve fibers and neurophysiologic control with the motor and somatosensory evoked potentials provide critical feedback to control neural thermal exposure.
On the other hand, we have the active neuroprotection techniques; it mainly involves different manoeuvres that help regulate the temperature in the region of interest. This is mainly achieved with the injection of warm or cool sterile fluid in an effort to normalise the temperature of the structure at risk. Pneumodissection can be also applied, though with CO2 we can create an insulation blanket and a safety distance between the ablation zone and the nerve at risk, but we can not cool down/warm up the nerves. Additionally, modifying the ablation parameters to optimize energy delivery (lower power settings and shorter exposure durations) can minimize the nerve damage.
Last but not least, in my talk I will provide a comparison of the risk of permanent neural damage between the different thermal ablation technologies, and examine the different ‘damage control’ strategies; corticosteroids and physiotherapy can help neural recovery.
Looking ahead, advancements in artificial intelligence-assisted imaging, biomaterials for nerve shielding, and tissue specific energy delivery algorithms promise to further refine neuroprotection strategies in percutaneous thermal ablation. Furthermore, ongoing research into regenerative therapies can further improve patient’s recovery after neural damage.
To conclude, the combination of all real-time monitoring, advanced imaging, warming/cooling strategies, optimized ablation parameters protocols and ‘damage control’ treatments collectively enhance patient safety and improve treatment outcomes even in the most challenging locations.